ML Aggarwal ICSE Solutions for Class 9 Maths Chapter 2 Compound Interest
ML Aggarwal SolutionsICSE SolutionsSelina ICSE Solutions

























































ML Aggarwal SolutionsICSE SolutionsSelina ICSE Solutions

























































ML Aggarwal SolutionsICSE SolutionsSelina ICSE Solutions







































ICSE SolutionsSelina ICSE Solutions
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 9 Chemistry Chapter 5 The Periodic Table. You can download the Selina Concise Chemistry ICSE Solutions for Class 9 with Free PDF download option. Selina Publishers Concise Chemistry for Class 9 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Chemistry Chapter 8 The Periodic Table
Page No. 79
Question 1.
What is the need for classification of elements?
Solution:
It is impossible for a chemist to study all the elements and their compounds. Hence, classification is a must.
Following are the reasons for the classification of elements:
Question 2.
What was the basis of the earliest attempts made for classification and grouping of elements?
Solution:
The first classification of elements was into 2 groups-metals and non-metals.
Question 3(a).
A, B and C are the elements of a Dobereiner’s triad. If the atomic mass of A is 7 and that of C is 39, what should be the atomic
mass of B?
Solution:
(a)
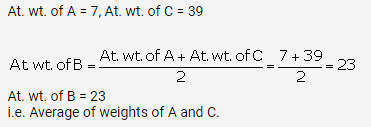
Question 3(a).
Why was Dobereiner’s triad discarded?
Solution:
(b) Döbereiner failed to arrange all the known elements in the form of triads.
In the triad of fluorine (19), chlorine (35.5) and bromine (80), it is observed that the mean of the atomic masses of fluorine and bromine is ½(19 + 80) = 49.5, not 35.5.
Question 4.
Explain ‘Newland’s Law of Octaves.’ Why was the law discarded?
Solution:
Elements when arranged in the increasing order of their atomic weights are similar to the eighth and the first note of the musical scale. For example, the eighth element from lithium is sodium. Similarly, the eighth element from sodium is potassium. Thus, lithium and sodium provide any specific place for hydrogen.
Question 5.
Did Dobereiners triads also exist in the columns of Newland’s Octaves? Compare and find out.
Solution:
Yes, Döbereiner’s triads also exist in the columns of Newland’s octaves. For example, the second column of Newlands classification has the elements Lithium (Li), Sodium (Na) and Potassium (K), which constitute a Döbereiner’s triad.
Question 6(a).
Lithium, sodium and potassium elements were put in one group on the basis of their similar properties. What are those similar properties?
Solution:
(a) Elements of lithium, sodium and potassium have the following similar properties:
Question 6(b).
The elements calcium, strontium and barium were put in one group or family on the basis of their similar properties.
What were those similar properties?
Solution:
(b)
Question 7(a).
What was Mendeleev’s basis for classification of elements?
Solution:
(a) Mendeleev’s basis for periodic classification:
Question 7(b).
Mendeleev’s contributions to the concept of periodic table laid the foundation for the Modern Periodic Table. Give reasons.
Solution:
(b) Mendeleev laid the foundation for the modern periodic table by showing periodicity of the properties of the elements by arranging the elements (63) then known into 8 groups, by leaving gaps for undiscovered elements and predicting their properties. He made separate groups for metals and non-metals. He also created periods in which the element gradually changes from metallic to non-metallic character. He was also able to show that the element in the same sub-group had the same valency.
Question 8.
State Mendeleev’s periodic law.
Solution:
Mendeleev’s periodic law: The physical and chemical properties of all the elements are a periodic function of their atomic masses.
Question 9(a).
Use Mendeleev’s Periodic Table to predict the formula of hydrides of carbon and silicon.
Solution:
(a) C is in Group 4. So, the hydride will be CH4 (Methane).
Si is in Group 4. So, the hydride will be SiH4 (Silane).
Question 9(b).
Use Mendeleev’s Periodic Table to predict the formula of oxides of potassium, aluminium and barium.
Solution:
(b)
K is in Group 1. So, the oxide will be K2O (Potassium oxide).
Al is in Group 3. So, the oxide will be Al2O3 (Aluminium oxide).
Ba is in Group 2. So, the oxide will be BaO (Barium oxide).
Question 10.
Which group of elements was missing from Mendeleev’s original periodic table?
Solution:
Anomalous pairs of elements were missing from Mendeleev’s periodic table.
Question 11.
State the merits of Mendeleev’s classification of elements.
Solution:
Merits of Mendeleev’s classification of elements:
Question 12.
Why did Mendeleev’s leave some gaps in his periodic table os elements? Explain your answer with an example.
Solution:
He left gaps in the table for the undiscovered elements. He discovered the properties of such elements with the help of neighboring elements.
He discovered eka-silicon with atomic mass of 72 which was later named Germanium with atomic mass 72.6.
Question 13.
The atomic number of an element is more important to the chemist than its relative atomic mass. Why?
Solution:
Henry Moseley found that when cathode rays struck anodes of different metals, the wavelength of these metals was found to decrease in a regular manner of changing the metal of anode in the order of its position in the periodic table. By this, he concluded that the number of positive charges present in the nucleus due to protons (atomic number) is the most fundamental property of the element.
So, Henry Moseley found that the atomic number is a better fundamental property of an element compared to its atomic mass. This lead to the modern periodic law.
This law gave explanations for anomalies in Mendeleev’s classification of elements such as
Question 14.
Consider the following elements: Be, Li, Na, Ca, K. Name the elements of (a) same group (b) same period.
Solution:
| Element | At. No. | Electronic distribution |
| Be | 4 | 2, 2 |
| Li | 3 | 2, 1 |
| Na | 11 | 2, 8, 1 |
| Ca | 20 | 2, 8, 8 |
| K | 19 | 2, 8, 8, 2 |
Question 15(a).
Name an element whose properties were predicted on the basis of its position in Mendeleev’s periodic table.
Solution:
a. Eka-silicon
Question 15(b).
Name two elements whose atomic weights were corrected on the basis of their positions in Mendeleev’s periodic table.}
Solution:
b. Gold and Platinum
Question 15(c).
How many elements were known at the time of Mendeleev’s classification of elements?
Solution:
c. Only 63 elements were discovered at the time of Mendeleev’s classification of elements.
Page No. 86
Question 1(a).
State the modern periodic law.
Solution:
a. Modern periodic law: The physical and chemical properties of all elements are a periodic function of their atomic numbers.
Question 1(b).
How many periods and groups are there in the modern periodic table?
Solution:
b. Eighteen groups and seven periods
Question 2.
What is the main characteristic of the last elements in the periods of a periodic table? What is the general name of such elements?
Solution:
Last elements of each period have their outermost shell complete, i.e. 2 or 8 electrons.
The general name is inert gases or noble gases.
Question 3(a).
What is meant in the periodic table by a group?
Solution:
a. Vertical columns in a periodic table which have the same number of valence electrons and similar chemical properties are called a group.
Question 3(b).
What is meant in the periodic table by a period?
Solution:
b. In a periodic table, elements are arranged in the order of increasing atomic numbers in horizontal rows called periods.
Question 4.
From the standpoint of atomic structure, what determines which elements will be the first and which the last in a period of the periodic table?
Solution:
Atomic number determines which element will be the first and which will be the last in a period of the periodic table.
Question 5(a).
What are the following groups known as?
Solution:
(a)
Question 5(b).
Name two elements of each group.
Solution:
(b)
Question 6(a).
What is the number of elements in the 1st period?
Solution:
(a) There are two elements in the first period.
Question 6(b).
What is the number of elements in the 3rd period, of the modern periodic table?
Solution:
(b) There are eight elements in the third period.
Question 7(a).
How does number of (i) valence electrons (ii) valency; vary on moving from left to right in the second period of a periodic table?
Solution:
(a)
Question 7(b).
How does number of (i) valence electrons (ii) valency; vary on moving from left to right in the third period of a periodic table?
Solution:
(b)
Question 8.
How do atomic structures (electron arrangements) change in a period with increase in atomic numbers moving left to right?
Solution:
The size of atoms decreases when moving from left to right in a period. Thus, in a particular period, the alkali metal atoms are the largest and the halogen atoms are the smallest.
Question 9(a).
This question refers to elements of the periodic table with atomic numbers from 3 to 18. In the table below, some elements are shown by letters, even though the letters are not the usual symbols of the elements.
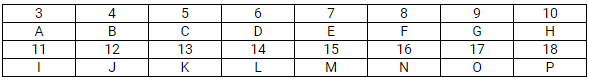
Which of these is:
Solution:
(a)
Question 9(b).
This question refers to elements of the periodic table with atomic numbers from 3 to 18. In the table below, some elements are shown by letters, even though the letters are not the usual symbols of the elements.

If A combines with F, what would be the formula of the resulting compound?
Solution:
(b) Li2O. A stands for lithium and F stands for oxygen. The valence of lithium is +1 and the valence of O is -2, i.e. A2F.
Question 9(c).
This question refers to elements of the periodic table with atomic numbers from 3 to 18. In the table below, some elements are shown by letters, even though the letters are not the usual symbols of the elements.

Solution:
(c) G has atomic number 9; therefore, its electronic arrangement is 2, 7.
Question 10.
Sodium and aluminium have atomic numbers 11 and 13, respectively. They are separated by one element in the periodic table, and have valencies 1 and 3 respectively. Chlorine and potassium are also separated by one element in the periodic table (their atomic numbers being 17 and 19, respectively) and yet both have valency 1. Explain.
Solution:
Na and Al have the capacity to donate an electron due to which the valency is positive, whereas Cl and K can only gain or lose one electron due to which their valency is -1 and +1, respectively. This is the only difference between these two.
Question 11.
Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common.
Solution:
These elements have a full outermost subshell, which results in high stability. They only react with other elements in extreme circumstances.
Question 12(a).
In which part of a group would you separately expect the elements to have the greatest metallic character?
Solution:
a. The greatest metallic character can be expected at the bottom of the group.
Question 12(b).
In which part of a group would you separately expect the elements to have the largest atomic size?
Solution:
b. The largest atomic size can be expected at the lower part of the group.
Question 13.
What happens to the number of valence electrons in atoms of elements as we go down a group of the periodic table?
Solution:
The number of valence electrons remains the same as we go down a group.
Question 14(a).
The position of elements A, B, C, D and E in the periodic table are shown below:
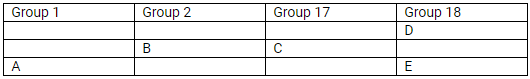
State which are metals, non-metals and noble gas in this table.
Solution:
(a) Metals: A and B; Non-metals: C; Noble gases: D and E
Question 14(b).
The position of elements A, B, C, D and E in the periodic table are shown below:

State which is most reactive (i) metal (ii) non-metal
Solution:
(b) Most reactive
Question 14(c).
The position of elements A, B, C, D and E in the periodic table are shown below:

Which type of ion will be formed by element A, B and C.
Solution:
(c)
Element A will form a positive ion 1+ (cation).
Element B will form a positive ion 2+ (cation).
Element C will form a negative ion 1– (anion).
Question 14(d).
The position of elements A, B, C, D and E in the periodic table are shown below:

Which is larger in size (i) D or E (ii) B or C
Solution:
(d)
Question 15.
Write the electronic configuration of element 17T35.
Solution:
K L M
Electronic configuration = 2, 8, 7
a. VIIA
b. Third period
c. Seven
d. Valency of T = -1
e. Non-metal
f. Protons = 17, Neutrons = 18
Page No. 91
Question 1.
Element P has atomic number 19. To which group and period, does P belong? Is it a metal or a non-metal? Why?
Solution:
Atomic number of P = 19
Electronic configuration = 2, 8, 8, 1
Group number of the element = 1
A Period number of the element = 4
P is a metal.
Question 2.
An element belongs to the third period and Group IIIA (13) of the periodic table. State:
a. the number of valence electrons,
b. the valency,
c. if it is a metal or non-metal?
d. the name of the element.
Solution:
a. 3
b. +3
c. Metal
d. Aluminium
Question 3.
Name and state the following with reference to the elements of the first three periods of the periodic table.
Solution:
(a) Helium
(b) Silicon
(c) 4, 3
(d) Argon
(e) Noble gases
(f) Carbon tetrachloride (CCl4)
(g) Silicon, Phosphorus
(h) Sodium chloride (Na+Cl–)
(i) Li and Mg; Be and Al; B and Si
(j) Sodium
(k) Typical elements of Period 2 belonging to group 14 and 15 are carbon and nitrogen.
Typical elements of’ Period 3 belonging to group 14 to 15 are silicon and phosphorus.
(l) Beryllium
Question 4.
Match column A with column B

Solution:
| Column A | Column B |
| (a) Elements short of 1 electron in octet | (v) Halogens |
| (b) Highly reactive metals | (iii) Alkali metals |
| (c) Unreactive elements | (ii) Noble gases |
| (d) Elements of groups 3 to 12 | (i) Transition elements |
| (e) Radioactive elements | (vi) Actinides |
| (f) Elements with 2 electrons in outermost orbit | (iv) Alkaline earth metals |
Question 5.
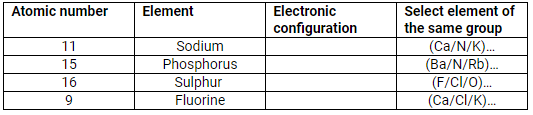
Complete the table:
Solution:
| Atomic number | Element | Electronic configuration | Select element of the same group |
| 11 | Sodium | 2, 8, 1 | K |
| 15 | Phosphorus | 2, 8, 5 | N |
| 16 | Sulphur | 2, 8, 6 | O |
| 9 | Fluorine | 2, 7 | Cl |
Question 6.
Solution:
a. Relative atomic mass of a light element up to calcium is approximately 20 its atomic number.
b. The horizontal rows in a periodic table are called periods.
c. Going across a period left to right, atomic size increases.
d. Moving left to right in the second period, number of valence electrons increases from 1 to 8.
e. Moving down in the second group, number of valence electrons remain same.
Question 7(a).
Name the alkali metals, How many electron(s) they have in their outermost orbit.
Solution:
Name of the alkali metals: Lithium, sodium, potassium, rubidium, cesium and francium
Electrons in the outermost orbit: 1
Question 7(b).
Take any one alkali metal and write its reaction with (i)oxygen (ii)water (iii)acid.
Solution:
Page No. 92
Question 8(a).
Name the method by which alkali metals can be extracted.
Solution:
a. Alkali metals can be extracted by the electrolysis of their molten salts.
Question 8(b).
What is the colour of the flame of sodium and potassium?
Solution:
b. The colour of the flame of sodium is golden yellow, and the colour of the flame of potassium is pale violet.
Question 9(a)
Name the first three alkaline earth metals.
Solution:
a. The first three alkaline earth metals are Beryllium, Magnesium and Calcium.
Question 9(b)
Write the reactions of first three alkaline earth metals with dilute hydrochloric acid.
Solution:
b. Reactions of the first three alkaline earth metals with dilute hydrochloric acid:
Be + 2HCl → BeCl2 + H2
Mg + 2HCl → MgCl2 + H2
Ca + 2HCl → CaCl2 + H2
Question 10(a)
How do alkaline earth metals occur in nature?
Solution:
a. Alkaline earth metals occur in nature in the combined state and not in the free state as they are very reactive.
Question 10(b)
Write the electronic configuration of the first two alkaline earth metals.
Solution:
b. Electronic configuration of the first two alkaline earth metals:
4Be: 1s22s2
12Mg: 1s22s22p63s2
Solution 11.
a. Group 17 elements are called halogens. The name halogens is from Greek halo (sea salt) and gens (producing, forming) and thus means ‘sea salt former’.
b. Group 17 elements or halogens:
Solution 12.
(a)
(b) Group 17 elements are highly reactive because of their closeness to the noble or stable gas configuration. They can easily achieve a noble gas electron structure.
Solution 13.
a. All the noble or inert gases have 8 electrons in their valence shell except helium which has two electrons in its valence shell.
b. Xenon or krypton from Group 18 can form compounds.
Solution 14.
Question 9.
An element A has 2 electrons in its fourth shell. State:
Solution 15.
a. Ca
b. 1s22s22p63s23p64s2
c. 2
d. Group 2 Period 4
e. Metal
f. Reducing agent
More Resources for Selina Concise Class 9 ICSE Solutions
ICSE SolutionsSelina ICSE Solutions
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Chemistry Chapter 7 Atomic Structure
Exercise 7
Solution 1.
The latest research on atom has proved that most of the postulates of Dalton’s atomic theory contradict. But Dalton was right that atoms take part in chemical reactions. Comparison of Dalton’s atomic theory with Modem atomic theory.
| Dalton’s atomic theory | Modern atomic theory |
| 1. Atoms are indivisible particles. | 1. Atoms are divisible into sub-atomic particles like protons, neutrons and electrons. |
| 2. Atoms can neither be created nor destroyed. | 2. Atoms can be created and destroyed by nuclear fusion and fission. |
| 3. The atoms of an element are alike in all respect and differ from atoms of other elements. | 3. The atoms of an element may not be alike in all respects, as it is seen in the case of isotopes. Isotopes which are atoms of the same element having the same atomic number but different mass numbers. |
Solution 2.
(a) Inert elements: The elements which have complete outer most shell i.e. 2 or electrons. They ordinarily do not enter into any reaction.
(b) These exist as monoatoms because molecules of these elements contain only one atom.
(c) Valence electrons: The number of electrons present in the outermost shell or valence shell is known as valence electrons.
Solution 3.
The three isotopes of hydrogen differ only due to their mass number which is respectively 1,2 and 3 and named protium, deuterium and tritium.

Solution 4.
| Atomic number | Name with valency |
| 4 | – |
| 15 | A solid non-metal of valency 3. |
| 8 | A gas of valency 2. |
| 19 | A metal of valency 1. |
| 14 | A non-metal of valency 4. |
Solution 5.
| Atom | Atomic number | Atomic mass | No. of Protons | No. of Electrons | No. of Neutrons | Electronic configuration |
| (a) Sodium | 11 | 23 | 11 | 11 | 12 | 2, 8, 1 |
| (b) Chlorine | 17 | 35 | 17 | 17 | 18 | 2, 8, 7 |
| (c) Oxygen | 8 | 16 | 8 | 8 | 8 | 2, 6 |
| (d) Carbon | 6 | 12 | 6 | 6 | 6 | 2, 4 |

Solution 6.
The significance of the number of protons found in the atoms in each of different element is fixed its place in periodic table.
Solution 7.
Atomic numbers of –
| X | Y | Z |
| 6 | 9 | 12 |
| (2,4) | (2,7) | (2,8,2) |
(a) Y (2,7) forms Anion.
(b) Z (2, 8, 2) forms Cation.
(c) X (2,4) has four electrons in the valence shell.
Solution 8.
(a) X+1
(b) Oxidising agent, because it has ability to donate electron.
Solution 9.
(a) Mass number:The atomic mass number is defined as the sum of the number of protons and neutrons contained in the nucleus of an atom of that element. It is denoted by the symbol A.
(b) Ion: An atom or molecule that carries a positive or negative charge because of loss or gain of electrons.
(c) Cation: It is positively charged ion that is formed when an atom loses one or more electrons e.g. Na+, Hg2+, Ca2+ etc.
(d) Atom: It is defined as the smallest unit of matter which takes part in a chemical reaction.
(e) Element: It is a substance which cannot be split up into two or simpler substances by usual chemical methods of applying heat, light or electric energy. e.g. Hydrogen, Oxygen, Chlorine etc.
(f) Orbit: It is defined as a circular path around the nucleus in which electrons of the atom revolve.
Solution 10.
Atomic number = 2 Mass number = 4
Solution 11.
(a) (i) Atom E contains 7 protons.
(ii) Atom E has an electronic configuration 2, 7.
(b) Atom C Stands for 73Li
Atom D stands for 816O
Compound formula = Li2O
(c) Metals are: A and C Non-metals are: E, D, E
Solution 12.
No of electrons in M Shell = 2
Number of electrons in K and L shell will be 2, 8 and respectively.
Therefore, Electronic configuration will be: 2, 8, 2.
Atomic number = 2 + 8 + 2 = 12, Since, atomic number = Number of Protons
No of Protons = 12
Solution 13.

(a) (ii) Electronic configuration = 2, 8, 2
(b) Mass numbers are different of two isotopes of magnesium because of different number of neutron, that is, 12 and 14 respectively.
Solution 14.
Nucleons: Particles which constitute nucleus are called nucleons. Proton and neutrons are the nucleons.
At. weight of phosphorus = 31 Atomic number = 15
Total number of nucleons = 31 (No. of P + No. of N) No. of neutrons = 31 – 15 = 16
Electronic configuration
No. of electrons = 15 = 2,8,5

Solution 15.
(a) Atoms of the same elements differing in the number of neutrons in their nuclei are known as isotopes. Thus, isotopes of an element have the same atomic number but different atomic mass number.
The fundamental particles is Neutrons which differs.
Uses of isotopes:
(b)

Solution 16.
In chemical reactions only electrons take part. The chemical properties depend upon the electronic configuration.
The isotopes of element 1735Cl and 1737Cl have same atomic number and hence, the same configuration. So they have same chemical properties. These differ only in physical contents and weights because neutrons contribute to the mass of an atom.
1735Cl and 1737Cl have different number of neutrons 18 and 20 respectively.
Solution 17.
The atomic masses of the isotopes of chlorine are 35 and 37. However in any given sample of chlorine gas, the isotopes occur in approximate 3 : 1, 75% of Cl35, and 25% of Cl37. Therefore, the relative atomic mass or atomic weight of chlorine is 35.5.
Fractional atomic weight of chlorine

Solution 18.
(a) Atomic number: The number of protons present in the nucleus of an atom is the atomic number of that atom. It is represented by the symbol Z.
Atomic number (Z) = Number of protons (p)
(b)
| No. of Protons | No. of Electrons | No. of Neutrons | Atomic number | Mass number | |
| 35 Cl 17 |
17 | 17 | 18 | 17 | 35 |
| 37 Cl 17 |
17 | 17 | 20 | 17 | 37 |
(c) Electronic configuration of chlorine is 2, 8, 7.
Solution 19.
(a) Hydrogen
(b) Element of zero group i.e. He (Helium)
(c) Calcium (2, 8, 8, 2). Therefore, 2 electrons in valance shell. Hence valency is 2.
(d) Chlorine atoms: 1735Cl and 1737Cl
(e) K shell.
Solution 20.
(a) Physical properties depend on the Atomic mass and isotopes have different mass number. (A) i.e. they have different number of neutrons. So, isotopes have different physical properties.
(b) Argon does not react as, Argon has completely filled outer-most orbit. The atomic number of argon is 18. Therefore, electronic configuration is 2, 8, 8. There are 8 electrons in the outermost or valance shell. Therefore, argon does not react.
(c) Actual Atomic Mass is greater than mass number (P + N) since mass number is a whole number approximation of atomic mass unit. In fact Neutrons are slightly heavier than protons and atom includes the existence of over 200 sub-atomic particles.
(d) 1735Cl and 1737Cl are isotopes of chlorine element which differ in number of neutrons. Whereas chemical properties are determined by electronic configuration of an atom. Isotopes of an element are chemically alike.
Solution 21.
Element A
Atomic number = 7
Electronic configuration: = 2, 5
Valency of element A = 8-5 = (3–)
Element B
Electronic configuration 2,8,8 Valency of element B = Zero Element C
Number of Electrons 13 Electronic configuration: 2,8,3
Valancy of element C = 3+
Element D
Protons = 18 = electrons Electronic configuration: 2,8, 8
Valency of element D = Zero
Element E
Electronic configuration = 2, 8, 8, 1
Valency of element E = 1+
[ii] C and E are metals [iii] A is a Non-metal
[iv] B and D are inert gases. (A, C and E are not inert gases.)
Solution 22.
(a) C. Atomic nucleus
(b) A. 6
(c) C. 2,8,8,1
Solution 23.
Elements tend to combine with one another to attain the stable electronic configuration of the nearest noble gas/inert gas (Duplet or Octet)
(a) Sodium Chloride
The electronic configuration of Sodium is 2, 8, Thus, Sodium atom tends to lose one electron to attain the stable electronic configuration of neon gas 2, 8.
The electronic configuration of Chlorine is 2, 8, 7. Thus, Chlorine atom needs one electron to complete its octet and achieve the stable electronic configuration of Argon 2, 8, 8.
When sodium and chlorine atoms approach each other, the sodium atom, Na, loses an electron to form sodium ion, The cation, Na+, carries a single positive charge.
Na → Na+ + e–
The electron lost by sodium atom is transferred to the chlorine atom forming chloride ion, Cl–.
Cl + e– → Cl–
The sodium and chloride ions thus, achieve stable electronic configurations.

(b) Hydrogen H2
Hydrogen atom has one electron in its valence shell. Hydrogen atom needs one more electron to complete its duplet and attain the stable electronic configuration of Helium.
In case of hydrogen H2 molecule, each of the two hydrogen H atoms contributes one electron so as to have one shared pair of electrons. Both the hydrogen atoms attain stable duplet structure resulting in the formation of a single covalent bond [H – H] between them.
Both the hydrogen atoms have equal attraction for the electrons. Thus, the shared pair of electrons remains equidistant from both the atoms.

Solution 24.
| Element Symbol | Atomic Number | Mass Number | Number of Neutrons | Number of Electrons | Number of Protons |
| Li | 3 | 6 | 4 | 3 | 3 |
| Cl | 17 | 35 | 18 | 17 | 17 |
| Na | 11 | 23 | 12 | 12 | 11 |
| Al | 13 | 27 | 14 | 13 | 13 |
| S | 15 | 32 | 16 | 15 | 15 |
ICSE SolutionsSelina ICSE Solutions
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Chemistry Chapter 3 Elements, Compounds and Mixtures
Exercise 3(A)
Solution 1.
An element is a pure substance composed of only one kind of atom. Example: C, H, O, Na, Ca, N etc.
Characteristics of an element:
Solution 2.

Solution 3.
Two elements which show exception to the properties of:
Metals :-
Non-metals:-
Solution 4.
(a) Molecule: A molecule is the smallest particle of a pure substance (element or compound), and it has all the properties of that substance. It is composed of atoms. It is capable of existing in a free state.
Example: O2, H2, Cl2 are molecules.
(b) Atomicity: Atomicity is the number of atoms present in a molecule of an element.
(c) Compound: A compound is a pure substance composed of two or more elements combined chemically in a fixed proportion by mass. The properties of compounds are different from the properties of their constituent elements. Example: H2O, CO2 etc.
Solution 5.
| (a) A diatomic element | Nitrogen (N2) |
| (b) A tetratomic element | Phosphorus (P) |
| (c) Monoatornic element | Helium (He) |
| (d) Lustrous non-metal | Iodine |
| (e) Liquid non-metal | Bromine (Br2) |
| (f) A gas filled in electric bulbs | Argon (Ar) |
| (g) A liquid metal | Mercury (Hg) |
| (h) A non metal conductor of electricity | Graphite |
| (i) A metal non malleable and non ductile | Zinc (Zn) |
| (j) A lustrous non metal | Graphite |
Solution 6.
(i) Sodium chloride is obtained when sodium chemically combines with chlorine in ratio of 23:355 by weight.
(ii) When molten sodium chloride is subjected to electrolysis, the ratio by weight of sodium and chlorine librated at electrodes is 2:3.
Solution 7.
| Type | Substances | Reason |
| Element | Chlorine, Sulphur | They cannot be split up into any simpler substance. |
| Compound | Carbon dioxide | It can be produces by chemical analysis of two or more simpler substances with different properties. |
| Mixture | Honey, milk, sea water, gun powder, apple juice, brine, syrup and bronze | These are produced by mere mixing of two or more substances in any proportions by weight. |
Solution 8.
(a) This is because molecules have all the properites of that substance and is capable of existing in a free state, molecules are composed of atoms.
(b)
| Element | Compund |
| 1. It is a pure substance which cannot be converted into simpler substances by any physical or chemical means. | 1. It is a pure substance made up two or more elements combined chemically in a fixed ratio. |
| 2. It is made up of only one kind of atoms. | 2. It is made up of two or more different kinds of atoms. |
| 3. The molecules are made up of one or more atoms. | 3. The molecules are made up of two or more atoms. |
Solution 9.
It is true that the elements can form different compounds.
Example: Hydrogen and oxygen combine to give two different compounds, water (H2O) and hydrogen peroxide (H2O2) under different conditions.
Solution 10.
Characteristics of a compound
Solution 11.
The properties of compounds are different from the properties of their constituent elements. Example: H2O, FeS, C12H22O11
Solution 12.
A compound is a pure substance composed of two or more elements combined chemically in fixed proportions by mass. The properties of a compound are different from the properties of their constituent elements.
Solution 13.
A mixture cannot be represented by a chemical formula because constituents present in a mixture are in any ratio and they are not chemically united.
Solution 14.
(a) Air
(b) Concrete
(c) Milk
Solution 15.
| Elements | Compounds | Mixtures |
| Mercury | Sugar, Distilled water, Alcohol, Nitre, Washing soda, Rust, Marble | Air, Milk, Wax, Sea-water, Paint, Brass, Bread, Soap, Tap water |
Solution 16.
On adding sulphuric acid to water we will get a Homogeneous Mixture (true solution).
This mixture will have different densities and boiling points depending upon the amounts of acid and water. The properties of acid and water will remain same even after mixing.
Solution 17.
Iron and sulphur when mixed, forms a mixture. It can be identified as follows:
It can be identified as follows:
Solution 18.
| Mixture | Compound |
| 1. It is obtained by the physical combination of either elements, or compounds, or both. | 1. It is obtained by the chemical combination of more than one element. |
| 2. The composition of elements present in a mixture is not fixed. | 2. The composition of elements present in a compound is fixed. |
| 3. It shows the properties of all its constituent elements. | 3. The properties of a compound are different from those of its elements. |
| 4. Its constituents can be separated using physical methods. | 4. Its constituents can be separated by using only chemical and electrochemical methods. |
| 5. The mixtures can be homogeneous or heterogeneous. | 5. A compound is always homogeneous in nature. |
Solution 19.
| No. | Types of mixture | Example | Nature |
| 1. | Two solids | Bronze (Zn, Cu, Sn) | Homogeneous |
| 2. | A solid in liquid | Sugar in water, Salt in water, Iodine in alcohol
Sugar in oil |
Homogeneous
Heterogeneous |
| 3. | Liquids | Oil in water, Kerosene in water,
Acetone + water |
Heterogeneous Homogeneous |
| 4. | Liquid and gas | Moisture in air | Homogeneous |
Solution 20.
| Homogeneous mixtures | Heterogeneous mixture |
| 1. A mixture is said to be homogeneous if its constituents are uniformly distributed and are not physically distinct. | 1. A mixture is said to be heterogeneous if its constituents are not uniformly distributed and are physically distinct. |
| 2. They have the same composition and properties throughout their mass. | 2. They have the different composition and properties in different parts of their mass. |
| 3. For example: sugar solution, salt in water etc. | 3. For example: Mixture of sand in water, Mixture of oil in water |
Solution 21.
(a)
(b) Air is considered as mixture not a compound because:
(c) Tap water does not have a fixed melting point. It may have various dissolved as well as undissolved impurities. Also it does not have a fixed composition.
Solution 22.
A pure substance is a homogeneous material with a definite, invariable chemical composition, and definite, invariable physical and chemical properties.
Sugar is a pure substance as all sample of sugar consists of same chemical composition. i.e. carbon, hydrogen and oxygen, C12H22O11.
Solution 23.
If dilute hydrochloric acid is added to the compound formed of iron and sulphur, Hydrogen sulphide gas is produced. (With a rotten egg smell).
Solution 24.


Solution 25.
When a mixture of powdered iron and sulphur is heated in a test tube, the powder starts melting and a pungent smell of a gas is given out. A dark grey solid is produced and this is called iron sulphide.
Solution 26.
Solute: A substance which gets dissolved in a solvent is called as solute.
Solvent: A substance in which solute gets dissolved in it is called as solvent.
Solution: A homogeneous mixture of two or more substances which are chemically non reacting, whose composition can be varied within certain limits is called a solution.
Solution = Solute + Solvent
Solution 27.
A homogeneous solution in which the size of the particle is about 10-10 m is called a true solution.
Properties of true solution:
Solution 28.
Properties of suspension are:
Solution 29.
A homogeneous looking heterogeneous mixture in which particles having a size between 10-10 m and 10-7 m dispersed in a continuous medium is called as colloid.
| Sr. no. | Dispersion medium | Dispersed phase | Common name of the system | Examples |
| 1. | Solid | Gas | Solid foam | Pumice stone, foam rubber etc. |
| 2. | Liquid | Gas | Foam or froth | Soap lather, shaving cream foam, lemonade froth, etc. |
| 3. | Liquid | Liquid | Emulsion | Milk, emulsified oils, medicines etc. |
| 4. | Solid | Liquid | Gel | Cheese, Butter, Jams, Jellies, Boot polish etc. |
| 5. | Gas | Liquid | Aerosol of liquids | Fog, mist, cloud, Liquid sprays etc. |
| 6. | Solid | Solid | Solid sol | Glasses, Gems, Pigmented plastics etc. |
| 7. | Liquid | Solid | Sol | Sulphur sol, Gold sol, Ferric hydroxide sol, Starch solution, Muddy water etc. |
| 8. | Gas | Solid | Aerosol of solids | Smoke, dust etc. |
Solution 30.
The movement of colloidal particles towards a particular electrode under the influence of an electric field is called electrophoresis.
It is shown by only those solutions, the particles of which carry a charge, such as colloid, particles. Thus this phenomenon of electrophoresis can be used to find the nature of charge carried by colloidal particles in a colloidal system.
For example, If the colloidal particles carry positive charge, they move. Towards negatively charge electrode (cathode) when subjected to an electric field. If they carry negative charge, they move towards positively charged electrode (Anode).
Solution 31.
| Sr no. | Property | True solution | Suspension | Colloids |
| 1 | Particle size | Less than 10-10 m | Greater than 10-7m | Between 10-10 to 10-7 m |
| 2 | Filtrability | Pass easily through ordinary filter paper as well as animal membranes. | Do not pass even through ordinary filter filter paper animal membranes. | Pass easily through ordinary paper but not through animal membranes. |
| 3 | Visibility of particles | Invisible | Visible to naked eye or under simple microscope. | Visible under Ultra microscope. |
Solution 32.
A suspension is a heterogeneous mixture in which very fine particles, about 10-7 m, of a solid are dispersed in any medium like a liquid or a gas.
Dispersed substance:
Examples:
Chalk in water, Sand in water, Coagulated matter.
Solution 33.
Tyndall effect can be defined as, the scattering of beam of light by colloidal particles present in a colloidal solution.
Tyndall effect can be observed when a fine beam of light passes through a small hole in the dark room. This happens due to the scattering of light by the particles of dust or smoke present in the air.
Experiment- In two Separate jars, take (FeSO4) Ferrous sulphate solution (green colour) in A and milk solution diluted with water in B.
Focus a torch on these jars in dark place. Torch light will not be visible in Ferrous sulphate solution and will not show Tyndall Effect as FeSO4 solution is actually true solution whereas light will be visible in B jar containing milk solution which is colloidal or Emulsion.
Examples of Tyndall effect from our daily life :-
Solution 34.
The effect is called as the Tyndall effect.
Tyndall effect is shown by colloidal solution.
Properties of colloidal solution:
Solution 35.
(a) Liquid in water – e.g. Alcohol solution, ammonia solution.
(b) Non-aqueous solution – e.g. solution of iodine in alcohol- This is called Tincture iodine. (c) Solid in non-aqueous solvent – e.g. solution of stearicacid in ethanol.
Solution 36.
Common names of colloids:
Solution 37.
| Solution | Mixture | Compound |
| 1. The solute is not present in any fixed proportion but its composition is uniform. | The composition is not uniform. The component may be present in any proportion. |
The constituents of a compound are combined in a definite proportion. |
| 2. The solute can be recovered by evaporating the solvent, i.e. by physical means. | The components can be separated by ordinary physical means. | The components can be separated by chemical means only |
Solution 38.
The distributed substance in the solution is called as dispersed phase.
The medium in which distributed substance is dispersed is referred to as dispersion medium.
Solution 39.
Blood – colloidal solution Sugar solution – true solution Salt solution – true solution
Starch solution – colloidal solution Ink – colloidal solution
Solution 40.
Starch solution, milk, soap solution, blue vitriol will scatter light as these are colloidal solutions.
Exercise 3(B)
Solution 1.
The methods used to separate solid-liquid mixture are:
Solution 2.
(a) Sand and water are separated by Filtration. Since sand is insoluble in water and forms Heterogeneous mixture. Water dissolves sodium chloride. The solution is than filtered with the help of a filter paper. Sand gets collected as a residue on the filter paper. And the dissolved sodium chloride in water can later be evaporated.
(b) Salt from an aqueous salt solution – Salt can be separated from aqueous salt solution by the process of evaporation. For this the aqueous salt solution (i.e. salt in water) is taken in a beaker and heated over flame. The liquid i.e. water will get evaporated leaving behind salt.
(c) Pure water from salt water- Pure water can be separated from salt water by the process of distillation. Distillation is the process of converting a liquid into vapour by heating and the subsequent condensation of the vapour back into a liquid. Water evaporates and recondenses in pure form and it is collected in a receiver. The salt residue remains in the distillation flask.

(d) Tea leaves from prepared tea – Tea leaves can be separated from prepared tea by the process of filtration. For this purpose a sieve or mesh made of steel or nylon is taken through which prepared tea is passed, thereby leading to retaining of tea leaves on the mesh.
(e) Cream from milk – Cream can be separated from milk by the process of centrifugation. The mixture is taken in centrifuge tubes and placed in holders. The holders are then rotated rapidly. After sometime, the rotation is stopped and the centrifuge tubes are taken out. It is observed that separation of mixture takes place on the bases of density. i.e. the more denser components settles at the bottom and the other becomes the supernatant which can be separated. In case of mixture of cream and milk. Cream comes in the upper layer and is recovered from milk.
(f) Sugar from sugar solution – It is separated by crystallisation. Heat the solution to obtain a saturated solution. Filter the solution while hot and allowed to cool. Crystals of sugar will be formed which are collected.
(g) Dye from black ink – Dye from black ink is separated by the process of chromatography. In this the constituents of a mixture are separated by their absorption over an appropriate absorbing material.
Solution 3.
(a) Carbon – titrachloride and water form immiscible mixture and can be separated by Separating Funnel. Since, they are immiscible liquids and forms two distinct layers.
Carbon tetrachloride is heavier liquid with the density of 1.59 g/cm3. Water is lighter with the density of 1g/cm3. Thus, the denser liquid carbon tetra chloride forms a lower level in the funnel which can be separated out first. The remaining water can be collected in the separate beaker.

(b) Lead chloride and silver chloride can be separated by solvent extraction. For this the given mixture is dissolved in water. Silver chloride dissolves in water but lead chloride does not dissolve. The solution is filtered through a filter medium, lead chloride will be retained on the filter medium and the filtrate containing water and silver chloride is collected. Then the filtrate is evaporated to get silver chloride.
Solution 4.
The method of separation depends on both the type of mixture and the physical properties of its constituents. These are :
(i) The physical state of the constituents.
(ii) The differences in the physical properties of the constituents such as :
(a) boiling point, (b) melting point, (c) density, (d) magnetic properties, (e) ability to sublime, (f) volatility, (g) solubility in various solvents .
Solution 5.
It is the process of dissolving one of the components in a particular liquid when one of the components is soluble in water or in some other solvents and the other component is not.
For example, a mixture of iron filings and sulphur is separated by using carb disulphide as solvent A mixture of sodium chloride and chalk can also be separated by using water as the solvent for sodium chloride.
Solution 6.
(a) Mercury, silver
(c) Magnesium, sodium carbonate (NO2CO3)
(b) Calcium, lead
(d) Sodium
Solution 7.
(a) Sublimation: Sublimation is the change of state of matter from solid to gaseous state without passing through the liquid state.
For example, To separate the mixture of ammonium chloride and sodium chloride, sublimation is used. On heating ammonium chloride will change into vapour which will condense into a solid in the neck of the inverted funnel which can be scrapped off. Sodium chloride remains in the evaporating dish.
(b)
| Evaporation | Sublimation |
| 1. Evaporation is change of state matter from solid state to gaseous state.
2. Evaporation takes place below its boiling point by supply of heat. |
1. Sublimation is the change of state of matter from solid state to gaseous state. 2. Sublimation takes place below its melting point by supply of heat. |
Solution 8.
(a) Distillation is the process of converting a liquid into vapour (by heating) and the subsequent condensation of the vapour back into a liquid.
(b) Fractional distillation is a process which involves distillation and collection of fractions or different liquids boiling at different temperatures.
(c) Centrifugation is the method of separating solids from a liquid, where the mixture is homogeneous.
Solution 9.
(a) Two miscible liquids can be separated by-
(b) Yes, mixture of chloroform (B. P. 61C) and carbon-tetrachloride (B. P. 77C) can be separated by Fractional Distillation as difference in their B. P. is less than 30C. Chloroform has a lower boiling point 61oC, so, it distils out first. It is collected in a receiver, leaving behind carbon tetrachloride (having boiling point 77oC).
Modification: A fractionating G-Column is fitted over distilling flask.
(c) Homogeneous Mixtures of solid and liquid are separated by Centrifugation.
Uses of Centrifugation
Solution 10.
(a) Dissolve the mixture of carbon and sulphur in carbon disulphide. Sulphur gets dissolve in carbon disulphide. The undissolved solid (carbon) is removed by filtration.
The filtrate is evaporated to dryness in order to recover the soluble solid i.e. sulphur powder.
(b) (i) Separation of potassium chloride: Prepare the suspension of mixture in water. The potassium chloride dissolves, but neither carbon nor sulphur, Filter the suspension and collect clear filtrate of potassium chloride in a separate beaker. Evaporate the clear filtrate on low heat. The water evaporates leaving behind white potassium chloride.
(ii) Separation of sulphur: Dissolve the residue in carbon disulphide. Sulphur dissolves in carbon disulphide leaving behind carbon. Filter the suspension and collect clear filtrate. Evaporate the filtrate in shade. The carbon disulphide evaporates leaving behind sulphur.
(iii) Removal and purification of carbon: Wash the residue of carbon on filter paper with carbon disulphide, so as to remove any sulphur. Dry the residue in shade. Carbon disulphide evaporates leaving behind carbon.
Solution 11.
(a) Chromatography
(b) Sedimentation
(c) Evaporation
(d) Fractional distillation
Solution 12.
By Sublimation method
Ammonium chloride and potassium chloride are placed in an evaporating dish and covered with an inverted funnel. On heating ammonium chloride will change into vapour which will condense into a solid in the neck of the inverted funnel which can be scrapped off. Sodium chloride remains in the evaporating dish.
Solution 13.
When caustic soda is added to an aqueous solution of copper sulphate, a blue precipitate of Cu(OH)2 , is obtained. Cu(OH)2 will be separated from mixture by filtration.
Solution 14.
Three commercial materials obtained from fractional distillation of petroleum are as follows:
Use of Natural gases:
Use of Kerosene oil:
Use of lubricating oil:
Solution 15.
(a) By Sublimation: Heat the impure iodine in a china dish over a low flame. When violet fumes of iodine starts coming out. Place a cold inverted funnel over the china dish. The violet fumes condense on the cooler sides of funnel to form tiny crystals of pure iodine.
(b) By Magnetic Separation: Roll a strong horse shoe magnet on the mixture. The iron filings cling to the magnet, leaving behind c
Solution 16.
(a) Alcohol from a mixture of alcohol and water can be separated through fractional distillation. Alcohol liberates first because it has a lower boiling point than water.
(b) The components which are soluble in water but their solubilities are different.
For example, (i) Sodium nitrate and sodium chloride. (ii) Potassium chlorate and potassium chloride.
Solution 17.
(a) Nitre and common salt:
Take a beaker and prepare a saturated solution of the mixture in boiling water. On cooling, nitre will crystallize out while common salt will remain in solution. The process is repeated two or three times to separate the components completely.
(b) Ammonia and hydrogen:
The mixture of gases is bubbled through a Woulfe’s bottle containing the solvent i.e. water in which Ammonia dissolves. The insoluble gas component i.e. hydrogen passes out through the delivery tube and is collected in gas jar. The soluble gas component is obtained from its aqueous solution by boiling the solution.
(c) Powdered Chalk and Sugar:
Dissolve given mixture in water. Sugar dissolves but chalk does not as it is insolube chalk. Filter the mixture. Chalk is residue and is dried in the folds of filter paper. Filtrate is crystallized to get crystals of sugar.
(d) Sand, table salt:
Dissolve mixture in water. Salt dissolves but sand do not. Filter the solution to collect sand on filter paper. Then the filtrate is evaporated to get salt.
Iron filings and naphthalene:
By magnetic separation. Iron fillings get cling to the magnet and are separated. Remaining will be naphthalene.
Solution 18.
(a) Ingredients of gun-powder are – (nitre,sulphurand charcoal):
Dissolve mixture in water. Nitre dissolves but not sulphur and charcoal. Filter the mixture and collect the clear filtrate of nitre. Heat filtrate to crystallisation point. On cooling crystals of nitre separate out. Filter the crystals and dry them.
Dissolve the sulphur and charcoal in Carbon disulphide. Sulphur dissolves but charcoal does not. Filter the solution. Allow the filtrate to evaporate in shade. Carbon disulphide evaporates leaving behind sulphur. Charcoal is dried on filter paper.
(b) Sulphur, Sand and common salt:
Dissolve mixture in water. Common salt dissolves but sulphur and sand are insoluble in water. Filter the solution. sulphur and sand will get collected on the filter paper. Evaporate the solution to get common salt Collect the residue (Sand and Sulphur powder)and dissolve it in carbon disulphide, CS2. Sulphur dissolves in carbon disulphide but sand is insoluble in carbon disulphide. Filter the solution and evaporate it to get sulphur. Sand is dried on filter paper.
(c) Carbon dioxide and Carbon Monoxide:
When mixture is passed through a long tube having a number of porous partitions, Carbon monoxide will diffuse more rapidly as compared to Carbon dioxide. Thus if there is sufficient partitions, in the end, Carbon dioxide comes out.
(d) Water and Sugar:
Take a beaker half filled with water and dissolve as much sugar as you can in it, with constant stirring. Now heat the solution and go on adding sugar till it stops dissolving. Filter solution while it is hot. Allow the solution to cool. The crystals of pure sugar settle down at the bottom of the beaker.
(e) Sand and Iodine:
The mixture is placed in a china dish and an inverted dry funnel is placed over it, with its stem closed with cotton wool. It is then gently heated at a low flame. Iodine sublimes on the cooler side of funnel in the form of fine powder crystals. The residue left behind in the china dish is sand.
Solution 19.
Remove Iron using a Magnetic Separation:
Dissolve mixture in water. Sodium Chloride dissolves but not sulphur and charcoal. Filter the solution and allow the filtrate to evaporate to get Sodium Chloride. Now dissolve Sulphur and Charcoal in carbon disulphide solution, CS2. Sulphur dissolves but not charcoal. Filter the solution. Allow the solution to evaporate to get sulphur. Dry, charcoal in folds of filter papers.
Solution 20.
(a) solid-solid mixture:
(b) Solid-liquid mixture:
(c) liquid-liquid mixture:
Solution 21.
The mixture is separated by exploiting the following facts in the order given below:
Solution 22.
(a) Dissolve given mixture in hot water lead chloride dissolves but not lead sulphate.
Filter the mixture. Residue is lead sulphate. Dry it in the folds of filter paper.
(b) Dissolve given mixture in water, Na2CO3, dissolves but not ZnCO3. Filter it ZnCO3 is residue and is dried in the folds of filter paper.
(c) Separation of given mixture can be done by fractional distillation. Boiling point of Benzene is 80°C and is less as compared with toluene having boiling point 111°C. So, Benzene distills over first.
(d) PbCl2 from a mixture of PbCl2 and AgCl.
Dissolve given mixture in water. AgCl dissolves, but not PbCl2. Filter the solution.
Evaporate the filtrate to get PbCl2.
Solution 23.
(a) Solid to gaseous state.
(b) Iodine
Solution 24.
Dissolve given mixture in dilute nitric acid. Copper oxide dissolves, but not charcoal. Filter the mixture, residue is charcoal. Dry it in the folds of filter paper. Now heat the filtrate to get copper oxide.
Solution 25.
To separate nitrogen from the air:
Solution 26.
The two main components (oxygen and nitrogen) are separated from the air as follows:
Solution 27.
(a) Chromatogeaphy: The process of separation of different dissolved constituents or mixture by absorbing them over an appropriate adsorbent material is called chromatography.
(b) The principle on which this technique is based is the difference in the adsorption different substances on the surface of a solid medium.
(c) Chromatograms are the distinct coloured rings or zones which are formed due to separation of mixture containing coloured substances by the process of chromatography.
(d) Advantages of chromatography:
Solution 28.
(a) Centrifugation is used in diagnostic laboratories for testing blood/urine samples. It is also used for separation of cream from milk and butter from curd in dairies.
(b) Chromatography is used for purification of number of industrial products.
Solution 29.
The methods employed to separate two liquids are:
Solution 30.
The apparatus used to separate oil and water is separating funnel. In this the two immiscible liquids are separated on the basis of their density differences. Thus, the heavier liquid will settle at bottom and the lighter one will form upper laye

Solution 31.
(a) Magnetic separation
(b) Solvent extraction
(c) Fractional crystallization
(d) Chromatography
(e) Boiling
(f) Separating funnel
(g) Distillation
(h) Evaporation
(i) Solvent extraction
Solution 32.
Take a rectangular sheet of filter paper approximately 10 em x 8em. Mark a horizontal line on the sheet about 2 cm from the lower edge and mark a cross (x) near the middle of the line. Prepare a mixture by mixing red and blue inks nearly-in equal proportions. With the help of dropper put about 2 to 3 drops of ink mixture on the cross (x) marked on the line. Allow it to dry. Fix the paper to a cork in a tall jar so that its lower end rests at the bottom of the jar. Pour a mixture of water and alcohol into the jar.
(1 : 1) so that about a 2 cm of filter paper dips in water-alcohol mixture. Leave it for about one hour. Solvent rises slowly by capillary action and reaches the cross marked on line and then goes on rising higher. Two coloured lines are seen at different heights. When the difference in heights is appreciable, remove the paper from jar and let it dry. In this way we can separate coloured constituents present in a mixture of ink.

Solution 33.
(a) sublimation
(b) filtration
(c) immiscible, separating funnel
(d) sublimation
(e) methylated sprit
ICSE SolutionsSelina ICSE Solutions
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Chemistry Chapter 1 Matter and its Composition
Exercise 1
Solution 1.
(a) Melting point: The constant temperature at which a solid changes into liquid state by absorbing heat energy is called melting point.
(b) Boiling point: It is the temperature at which a liquid changes into vapour under atmospheric pressure.
(c) Evaporation: The slow passing of molecules of a liquid into gaseous state at a temperature below its boiling point.
(d) Freezing: It is a process in which a liquid changes into solid state by giving out heat energy.
Solution 2.
Boiling point of a liquid can be raised by increasing the atmospheric pressure.
Solution 3.
On heating, solid wax melts into liquid wax, which on further heating, is converted into wax vapours. These changes can be seen in a burning candle. The candle is made up of a solid wax. When we light a candle, the wax near its wick melts. The molten wax rises up the wick and is converted into wax vapour. The wax vapour mixes with oxygen in the air and burns. In a lighted candle, you can see the solid and the liquid states of wax. The vapour of wax can be seen rising from the wick for some time after the candle is put out.

Solution 4.
(a) Sublimation: The process by which a solid directly change to its vapour state (or gaseous state) without passing through liquid state and vice versa is called sublimation.
(b) Liquefaction: It is a process of change of state of a substance from gaseous state to liquid state at a particular temperature. It is also known as condensation.
(c) Melting: It is process of changing from solid state to a liquid state at a particular temperature.
(d) Boiling: The process by which a liquid rapidly changes into a gaseous state, by absorbing the heat energy is called boiling.
Solution 5.
(a)
| An atom | A molecule |
| Atom is a smallest particle of an element. | Molecule is a group of two or more atoms combined together so it is bigger. |
| Atom consists of nucleus (containing protons and neutrons) and electrons. | Molecule consists of combination of two or more like or different atoms chemically bound together e.g. H2, HCl, NaCl etc. |
| Atom can neither be seen through naked eye nor through magnifying microscope. | Molecule is not visible to naked eye, while can be seen through highly magnifying microscope. |
| Atom cannot be further divided. | Molecule can further be divided to give individual atoms. |
| Atoms may or may not have independent existence. | Molecules are capable of having independent existence. For example, atom of oxygen (O) has no independent existence while its molecule exists as O2in nature. |
(b)
| Boiling | Evaporation |
| Boiling is the process in which liquid gets converted into gaseous state. | Evaporation is a process in which the liquid gets converted into its gaseous form at any temperature below its boiling point. |
| Boiling occurs at the entire mass of the liquid. That is, it is a bulk phenomenon. | Evaporation occurs on the surface of the liquid. That is, it is a surface phenomenon. |
| Boiling occurs rapidly. | Evaporation is a slow process. |
| Boiling occurs at a specific temperature. | Evaporation occurs at any temperature. |
(c)
| Melting | Boiling |
| The process of changing from solid state to a liquid state at a particulartemperature is called melting or fusion. | The process of change of liquid to vapour form all parts of the liquid at a particular temperature is called boiling. |
| Melting refers to the phenomenon when a solid transforms into a liquid. | Boiling refers to the phenomenon when liquid transform into a gas. |
| Example: Melting of ice | Example: Boiling of water |
(d)
| Gas | Vapour |
| A substance exists as a gas at the room temperature and atmospheric pressure. | A substance is a solid or liquid under ordinary condition but it is gaseousunder specific conditions. |
| It is present at ordinary conditions of temperature. | Its temperature is lower than the boiling point of its liquid state. |
| e.g. – Nitrogen, oxygen. | e.g. – Iodine, Camphor |
Solution 6.
(a) Water boils of 100oC under 1 atmosphere pressure.
(b) At high altitude water boils below 100oC.
(c) A liquid evaporates below its boiling point.
(d) When a substance is heated kinetic energy of the particles increases.
(e) Solids have the negligible inter-particle space.
(f) Gases have the negligible inter-particle forces.
Solution 7.
(a) Increase in atmospheric pressure
(b) Sulphur
(c) Inter-conversion of state of matter
Solution 8.
(a) Sublimation
(b) Melting
(c) Evaporation
(d) Vaporisation
Solution 9.
(a) Increase in temperature favours Evaporation. When evaporation occurs, remaining liquid becomes cooler. The particles of the liquid absorb heat energy from surroundings to regain energy lost during evaporation which makes the surroundings cold.
(b) Earthen pot has pores which help in evaporation. Some of the water continuously seeps out from these pores. This water absorbs heat of vaporization from the remaining water and gets evaporate. Thus, the remaining water loses heat and gets cooled.
(c) This happens because, when the petrol changes from liquid state to the vapour state, is absorbs heat energy from the palm. The palm thus loses heat and gets cooled.
(d) In humid weather wet clothes take longer time to dry up due to the slow evaporation of water from their surface.
(e) Evaporation is a surface phenomenon. With increase in surface area, evaporation increases. Hot tea in Saucer cools faster than in a cup and hence we can sip faster.
Solution 10.
(a) Naphthalene balls become smaller day by day as they have very weak force of attraction operating between their particles, which break away from other particles from the surface of solid without heating.
(b) In gases the particles are far apart and there is enough space available for compression. Hence, gases can be compressed easily.
(c) Heat energy supplied increases the rate of vibration of the particles and decreases. The inter-particle attraction.
(d) Light has no mass and it does it occupy space. Thus, it is not considered as matter.
(e) According to ‘Law of Conservation of Mass’, “Mass can neither be created nor destroyed in a chemical reaction.” However, it may change from one form to other.
Solution 11.
In summers, we perspire more. Cotton being a good absorber of water helps in absorbing the sweat and exposes it to the atmosphere for evaporation. When sweat evaporates from our body, it takes heat from our body. The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool.
Solution 12.
Balloon get heat from sun and on heating, the vibration of particles increases and the inter-particle force of attraction between them gets reduced, therefore, balloon bursts.
Solution 13.
Law of conservation of mass: It states that mass can neither be created nor destroyed in a chemical reaction. During any change, physical or chemical, matter is neither created nor destroyed. However it may change from one form to another.
Experimental Verification of Law of Conservation of Mass
Requirements: H-shaped tube called Landolt’s tube, Sodium chloride solution, silver nitrate solution, etc.
Procedure: A specially designed H-shaped tube is taken. Sodium chloride solution is taken in one limb ofthe tube and silver nitrate solution in the other limb as shown in figure. Both the limbs are now sealed and weighed. Now the tubes is averted so that the solutionscan mix up together and react chemically. The reaction takes place and a white precipitate of silver chloride is obtained.

The tube is weighed again. The mass of the tube is found to be exactly the same as the mass obtained before inverting the tube. Thus, this experiment clearly verifies the law of conservation of mass

Solution 14.
Law of conservation of mass is applied to a burning candle. A candle is made of solid wax. When it is lighted, wax near its wick melts and changes into to liquid form. The molten wax rises up the wick and is converted into wax vapours. The wax vapours their mix with oxygen in the air.
Thus, in burning of candle the matter is neither created nor destroyed but one form is changed into the other form.
Solution 15.
The reaction is:

Total mass of reactants = (6 g + 5.3 g) = 11.3 g
Total mass of products = (8.2 + 2.2 + 0.9) g = 11.3 g
As the total mass of reactants is equal to the total mass of products. Hence, the reaction follows Law of conservation of mass.
Solution 16.
The reaction will be as follows:
Methane + Oxygen → Carbon dioxide + Water
According to law of conservation of mass,
Total mass of reactants = Total mass of products
Mass of methane + mass of oxygen = Mass of carbon dioxide + Mass of Water
Mass of methane + 32 g = 22 + 18 g
Mass of methane = (40 – 32) = 8 g
8g of methane is required.
Solution 17.
Word equation for the reaction is:
Sodium + Chlorine → Sodium Chloride
According to law of conservation of mass,
Total mass of reactants = Total mass of products
Mass of sodium + Mass of chlorine = Mass of sodium chloride
23 g+ Mass of chlorine = 58.5 g
Mass of chlorine = 58.5 – 23 = 35.5 g
35.5 g of chlorine is needed.
Solution 18.
Word equation for the reaction is:
Magnesium + Oxygen → Magnesium oxide
According to law of conservation of mass,
Total mass of reactants = Total mass of products
Mass of magnesium + Mass of oxide = Mass of magnesium oxide
4.8g + 3.2g = Mass of magnesium oxide
Mass of magnesium oxide = 8 g
Solution 19.
(a) (iv) No fixed shape and size highly compressible.
(b) (i) The solid starts melting.
(c) (i) evolved
(d) (iv) decreases with increasing pressure.
(e) (iii) Iodine
Solution 20.
(a) does not, melting, boiling
(b) liquid, solid
(c) temperature, temperature
(d) gaseous, sublimation
(e) high and negligible
Solution 21.
(a) freezing
(b) less
(c) sublimation
(d) remains constant
Solution 22.
| Column A | Column B |
| (a) Constituent of matter | Molecules |
| (b) No compressibility | Solid |
| (c) Maximum expansion | Gas |
| (d) Conversion of a gas into liquid | Condensation |
ICSE SolutionsSelina ICSE Solutions
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 9 Physics Chapter 5 Upthrust in Fluids, Archimedes’ Principle and Floatation. You can download the Selina Concise Physics ICSE Solutions for Class 9 with Free PDF download option. Selina Publishers Concise Physics for Class 9 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
Selina ICSE Solutions for Class 9 Physics Chapter 5 Upthrust in Fluids, Archimedes’ Principle and Floatation
Exercise 5(A)
Solution 1S.
When a body is partially or wholly immersed in a liquid, an upward force acts on it. This upward force is known as an upthrust.
Upthrust can be demonstrated by the following experiment:
Take an empty can and close its mouth with an airtight stopper. Put it in a tub filled with water. It floats with a large part of it above the surface of water and only a small part of it below the surface of water. Push the can into the water. You can feel an upward force and you find it difficult to push the can further into water. It is noticed that as the can is pushed more and more into the water, more and more force is needed to push the can further into water, until it is completely immersed. When the can is fully inside the water, a definite force is still needed to keep it at rest in that position. Again, if the can is released in this position, it is noticed that the can bounces back to the surface and starts floating again.
Solution 2S.
Buoyant force on a body due to a liquid acts upwards at the centre of buoyancy.
Solution 3S.
The property of a liquid to exert an upward force on a body immersed in it is called buoyancy.
Solution 4S.
The upward force exerted on a body by the fluid in which it is submerged is called the upthrust. Its S.I. unit is ‘newton’.
Solution 5S.
A liquid contained in a vessel exerts pressure at all points and in all directions. The pressure at a point in a liquid is the same in all directions – upwards, downwards and sideways. It increases with the depth inside the liquid.

When a body is immersed in a liquid, the thrusts acting on the side walls of the body are neutralized as they are equal in magnitude and opposite in direction. However, the magnitudes of pressure on the upper and lower faces are not equal. The difference in pressure on the upper and lower faces cause a net upward force (= pressure x area) or upthrust on the body.
It acts at the centre of buoyancy.
Solution 6S.
Upthrust due to water on block when fully submerged is more than its weight. Density of water is more than the density of cork; hence, upthrust due to water on the block of cork when fully submerged in water is more than its weight.
Solution 7S.
A piece of wood if left under water comes to the surface of water because the upthrust on body due to its submerged part is equal to its own weight.
Solution 8S.
Experiment to show that a body immersed in a liquid appears lighter:

Take a solid body and suspend it by a thin thread from the hook of a spring balance as shown in the above figure (a). Note its weight. Above figure (a) shows the weight as 0.67 N.
Then, take a can filled with water. Immerse the solid gently into the water while hanging from the hook of the spring balance as shown in figure (b). Note its weight. Above figure (b) shows the weight as 0.40 N.
The reading in this case (b) shall be less than the reading in the case (a), which proves that a body immersed in a liquid appears to be lighter.
Solution 9S.
The readings in the spring balance decreases.
As the cylinder is immersed in the jar of water, an upward force acts on it, which is in opposition to the weight component of the cylinder. Hence the cylinder appears to be lighter.
Solution 10S.
A body shall weigh more in vacuum because in vacuum, i.e. in absence of air, no upthrust will act on the body.
Solution 11S.
Upthrust on a body depends on the following factors:
Solution 12S.
Larger the volume of body submerged in liquid, greater is the upthrust acting on it.
Solution 13S.
A stone falls faster.
Because the volume of stone is less than the volume of bunch of feathers of the same mass, the upthrust due to air on stone is less than that on the bunch of feathers, and hence, the stone falls faster.
However, in vacuum, both shall fall together because there will be no upthrust.
Solution 14S.
F2 > F1; Sea water is denser than river water; therefore, the upthrust due to sea water will be greater than that due to river water at the same level. This shall make the body to appear lighter in the sea water.
Solution 15S.
Observation: Volume of a block of wood immersed in glycerine is smaller as compared to the volume of block immersed in water.
Explanation: Density of glycerine is more than that of water. Hence, glycerine exerts more upthrust on the block of wood than water, causing it to float in glycerine with a smaller volume.
Solution 16S.

Solution 17S.

Solution 18S.
(a) Both have equal volumes.
(b) Bounce back to the surface.
(c) More than
Solution 19S.

Consider a cylindrical body PQRS of cross-sectional area A immersed in a liquid of density ρ as shown in the figure above. Let the upper surface PQ of the body is at a depth h1 while its lower surface RS is at depth h2 below the free surface of liquid.
At depth h1, the pressure on the upper surface PQ,
P1 = h1 ρg.
Therefore, the downward thrust on the upper surface PQ,
F1 = Pressure x Area = h1 ρgA ……………….(i)
At depth h2, pressure on the lower surface RS,
P2 = h2 ρg
Therefore, the upward thrust on the lower surface RS,
F2 = Pressure x Area = h2 ρgA …………………(ii)
The horizontal thrust at various points on the vertical sides of body get balanced because the liquid pressure is the same at all points at the same depth.
From the above equations (i) and (ii), it is clear that F2 > F1 because h2 > h1 and therefore, body will experience a net upward force.
Resultant upward thrust or buoyant force on the body,
FB = F2 – F1
= h2 ρgA – h1 ρgA
= A (h2 – h1) ρg
However, A (h2 – h1) = V, the volume of the body is submerged in a liquid.
Therefore, upthrust FB = V ρg.
Now, V g = Volume of solid immersed x Density of liquid x Acceleration due to gravity
= Volume of liquid displaced x Density of liquid x Acceleration due to gravity
= Mass of liquid displaced x Acceleration due to gravity
= Weight of the liquid displaced by the submerged part of the body
Thus, Upthrust FB = weight of the liquid displaced by the submerged part of the body…..(iii)
Now, let us take a solid and suspend it by a thin thread from the hook of a spring balance and note its weight.
Then take a eureka can and fill it with water up to its spout. Arrange a measuring cylinder below the spout of the eureka can as shown. Immerse the solid gently in water. The water displaced by the solid is collected in the measuring cylinder.
When the water stops dripping through the spout, note the weight of the solid and volume of water collected in the measuring cylinder.
From the diagram, it is clear that
Loss in weight (Weight in air – Weight in water) = Volume of water displaced.
Or, Loss in weight = Volume of water displaced x 1 gcm-3 [Because the density of water = 1 gcm-3]
Or, Loss in weight = Weight of water displaced ……………(iv)
From equations (iii) and (iv),
Loss in weight = Upthrust or buoyant force
Solution 20S.
Since the spheres have the same radius, both will have an equal volume inside water, and hence, the upthrust acted by water on both the spheres will be the same.
Hence, the required ratio of upthrust acting on two spheres is 1:1.
Solution 21S.
Sphere of iron will sink.
Density of iron is more than the density of water, so the weight of iron sphere will be more than the upthrust due to water in it; thus, it causes the iron sphere to sink.
Density of wood is less than the density of water, so the weight of sphere of wood shall be less than the upthrust due to water in it. So, the sphere of wood will float with a volume submerged inside water which is balanced by the upthrust due to water.
Solution 22S.
The bodies of average density greater than that of the liquid sink in it. While the bodies of average density equal to or smaller than that of liquid float on it.
Solution 23S.
(i) The body will float if ρ ≤ ρL
(ii) The body will sink if ρ > ρL
Solution 24S.
It is easier to lift a heavy stone under water than in air because in water, it experiences an upward buoyant force which balances the actual weight of the stone acting downwards. Thus, due to upthrust there is an apparent loss in the weight of the heavy stone, which makes it lighter in water, and hence easy to lift.
Solution 25S.
Archimedes’ principle states that when a body is immersed partially or completely in a liquid, it experiences an upthrust, which is equal to the weight of liquid displaced by it.
Solution 26S.
Let us take a solid and suspend it by a thin thread from the hook of a spring balance and note its weight (Fig a).
Then take a eureka can and fill it with water up to its spout. Arrange a measuring cylinder below the spout of the eureka can as shown. Immerse the solid gently in water. The water displaced by the solid gets collected in the measuring cylinder.

When water stops dripping through the spout, note the weight of the solid and volume of water collected in the measuring cylinder.
From diagram, it is clear that
Loss in weight (Weight in air – weight in water) = 300 gf – 200 gf = 100 gf
Volume of water displaced = Volume of solid = 100 cm3
Because density of water = 1 gcm-3
Weight of water displaced = 100 gf = Upthrust or loss in weight
This verifies Archimedes’ principle.
Solution 1M.
Turpentine
Solution 2M.
N
Solution 3M.
ρ > ρL
Solution 1N.

Solution 2N.

Solution 3N.

Solution 4N.

Solution 5N.

Solution 6N.

Solution 7N.

Solution 8N.

Solution 9N.

Solution 10N.

Exercise 5(B)
Solution 1S.
The density of a substance is its mass per unit volume.
Solution 2S.
(i) The C.G.S. unit of density is gcm-3.
(ii) The S.I. unit of density is kgm-3.
Solution 3S.
1 gcm-3 = 1000 kgm-3
Solution 4S.
It means the mass of 1 m-3 of iron is 7800 kg.
Solution 5S.
Density of water at 4°C in S.I. units is 1000 kgm-3.
Solution 6S.
(i) Mass of a metallic body remains unchanged with increase in temperature.
(ii) Volume of metallic body increases with an increase in temperature.
(iii) Density (= Mass/volume) of a metallic body decreases with an increase in temperature.
Solution 7S.
On heating from 0°C, the density of water increases up to 4°C and then decreases beyond 4°C.
Solution 8S.
(i) Volume, (ii) kg m-3, (iii) 1000 and (iv) 1000
Solution 9S.
The relative density of a substance is the ratio of density of that substance to the density of water at 4°C.
Solution 10S.
Relative density is the ratio of two similar quantities; thus, it has no unit.
Solution 11S.
Density of a substance is the ratio of its mass to its volume but R.D. of a substance is the ratio of density of that substance to the density of water at 4°C.
Solution 12S.

Steps:
Observations:
Loss in weight of solid when immersed in water = (W1 – W2) gf
R.D. = Weight of solid in air/Loss of weight of solid in water
R.D. = W1/(W1 – W2).
If the solid is soluble in water, then instead of water, take a liquid in which the solid is insoluble and it sinks in the liquid.
Then, R.D. = (Weight of solid in air/Loss of weight of solid in liquid) x R.D. of the liquid
Solution 13S.

Solution 14S.
Experimental determination of R.D. of a solid lighter than water (such as cork):

Observations:
Weight of the sinker in water = W1gf
Weight of the sinker in water and cork in air = W2gf
Weight of sinker and cork together in water = W3gf
Calculations:
Weight of cork in air = (W2 – W1) gf
Weight of cork in water = (W3 – W1) gf
Loss in weight of the cork in water = Weight of cork in air Weight of cork in water.
= [(W2 – W1) – (W3 – W1)] gf
= (W2 – W3) gf
R.D. of cork = Weight of cork in air/Loss of weight of cork in water
Or, R.D. of cork = (W2 – W1)/(W2 – W3).
Solution 15S.
The weight of the sinker and cork combined, in water will be less than the weight of the sinker alone in water because the upthrust due to water on cork (when completely immersed) is more than the weight of cork itself.
Solution 1M.
Water
Solution 2M.
No unit.
Solution 3M.
1 g cm-3
Solution 1N.

Solution 2N.

Solution 3N.

Solution 4N.

Solution 5N.

Solution 6N.

Solution 7N.

Solution 8N.

Solution 9N.

Solution 10N.

Solution 11N.

Solution 12N.

Solution 13N.

Solution 14N.

Solution 15N.

Solution 16N.
a. The mass of stone is 15.1 g. Hence, its weight in air will be Wa = 15.1 gf
b. When stone is immersed in water its weight becomes 9.7 gf. So, the upthrust on the stone is 15.1 – 9.7 = 5.4 gf, Since the density of water is 1 g cm-3, the volume of stone is 5.4 cm3.
c. Weight of stone in liquid is Wl = 10.9 gf
Weight of stone in water is Ww = 9.7 gf
Therefore, the relative density of stone is

Exercise 5(C)
Solution 1S.
According to the principle of floatation, the weight of a floating body is equal to the weight of the liquid displaced by its submerged part.
Solution 2S.
(i) Two forces acting on the body are as listed below:
(a) Weight of the body (downwards)
(b) Upthrust of the liquid (upwards)

(ii) If the weight of the body is greater than the upthrust acting on it, the body will sink
If the weight of the body is equal to or less than the upthrust acting on it, the body will float.
(iii) (a) The net force acting on the body when it sinks is body’s own weight.
(b) The net force acting on the body when it floats is the upthrust due to the liquid.
Solution 3S.
The reading on the spring balance will be zero because wood floats on water and while floating the apparent weight = 0.
Solution 4S.
(a) The ball will float because the density of ball (i.e. iron) is less than the density of mercury.
(b) While floating, the apparent weight = 0.
Solution 5S.
The body will float if its density is less than or equal to the density of the liquid ρS ≤ ρL.
The body will sink if its density is greater than the density of the liquid ρS > ρL.
Solution 6S.
Density of iron is less than the density of mercury; hence, an iron nail floats in mercury and density of iron is more than the density of water; hence, an iron nail sinks in water.
Solution 7S.
(i) Weight of the floating body is equal to the upthrust.
(ii) While floating, the apparent weight is zero.
Solution 8S.
When the body is partially immersed, its centre of buoyancy will be below the centre of gravity of the block.
When the body is completely immersed, its centre of buoyancy will coincide the centre of gravity.
Solution 9S.
The upthrust on the body by each liquid is the same and equal to the weight of the body.
However, upthrust = Volume submerged × ρL × g,
For the liquid C, since the volume submerged is least so the density ρ3 must be maximum.
Solution 10S.

The forces acting are as listed below:
Weight of water displaced by the floating body = Weight of the body
Solution 11S.
Centre of buoyancy: It is the point through which the resultant of the buoyancy forces on a submerged body act; it coincides with the centre of gravity of the displaced liquid, if the body is completely immersed.
For a floating body with its part submerged in the liquid, the centre of buoyancy is at the centre of gravity of the submerged part of the body and it lies vertically below the centre of gravity of the entire body.
Solution 12S.
Observation : The balloon will sink.
Explanation : As air is pumped out from jar, the density of air in jar decreases, so the upthrust on balloon decreases. As weight of balloon exceeds the upthrust on it, it sinks.
Solution 13S.
(a) It will float with some part outside water.
Reason : On adding some salt to water, the density of water increases, so upthrust on a block of wood increases, and hence, the block rises up till the weight of salty water displaced by the submerged part of block becomes equal to the weight of the block.
(b) The block will sink.
Reason: On heating, the density of water decreases, so upthrust on the block decreases and the weight of block exceeds upthrust due to which it sinks.
Solution 14S.

Solution 15S.
Density of brine is more than the density of water. Hence, the upthrust exerted by brine is more than the upthrust exerted by water on ice. Therefore, floating ice is less submerged in brine.
Solution 16S.
(i) 1:1; The weight of the water displaced by the man in sea and river will be same and will be equal to his own weight.
(ii) He finds it easier to swim in the sea because the density of sea water is more than the density of river water. So his weight is balanced in sea water with a part of his body submerged in the water.
Solution 17S.
An iron nail sinks in water because density of iron is more than the density of water, so the weight of the nail is more than the upthrust of water on it.
On the other hand, ships are also made of iron, but they do not sink. This is because the ship is hollow and the empty space in it contains air, which makes its average density less than that of water. Therefore, even with a small portion of ship submerged in water, the weight of water displaced by the submerged part of ship becomes equal to the total weight of ship and it floats.
Solution 18S.
Due to the hollow and empty space in the ship, the average density of a ship is less than the density of water.
Solution 19S.
When a floating piece of ice melts into water, it contracts by the volume equal to the volume of ice pieces above the water surface while floating on it. Hence, the level of water does not change when ice floating on it melts.
Solution 20S.
Forces acting on the body are listed below:
Solution 21S.
A ship submerges more as it sails from sea water to river water.
Density of river water is less than the density of sea water. Hence, according to the law of floatation, to balance the weight of the ship, a greater volume of water is required to be displaced in river water of lower density.
Solution 22S.
(a) Icebergs are dangerous for ships as they may collide with them. Icebergs being lighter than water, float on water with a major part of their surfaces laying under the water surface and only a small part lies outside water. Thus, it becomes difficult for the driver of the ship to estimate the size of the iceberg.
(b) Density of a strong salt solution is more than the density of fresh water. Hence, the salt solution exerts a greater upthrust on the egg which balances the weight of the egg, so the egg floats in a strong salt solution but sinks in fresh water.
(c) Density of hydrogen is much less than the density of carbon dioxide. When a balloon is filled with hydrogen, the weight of the air displaced by an inflated balloon (i.e. upthrust) becomes more than the weight of a gas filled balloon, and hence, it rises. In case of a balloon filled with carbon dioxide, weight of the balloon becomes more than the upthrust of the air, and hence, it sinks to the floor.
(d) As a ship in harbor is unloaded, its weight decreases. As a result, it displaces less water, and the ship’s hull rises in water till the weight of the water displaced balances the weight of the unloaded ship.
(e) The reason is that the density of air decreases with altitude. Therefore, as the balloon gradually goes up, the weight of the displaced air (i.e. uphrust) decreases. It keeps on rising as long as the upthrust exceeds its weight. When upthrust becomes equal to its weight, it stops rising.
(f) Density of river water is less than the density of sea water. Hence, according to the law of floatation, to balance the weight of the ship, a great volume of water is required to be displaced in river water having a comparitively lower density.
Solution 1M.
W= FB
Solution 2M.
Zero
Solution 3M.
ρ1 > ρ2
Solution 1N.

Solution 2N.

Solution 3N.

Solution 4N.

Solution 5N.

Solution 6N.

Solution 7N.

Solution 8N.

Solution 9N.

More Resources for Selina Concise Class 9 ICSE Solutions
APlusTopper.com provides step by step solutions for Selina Concise ICSE Solutions for Class 10 Biology Chapter 3 Genetics Some Basic Fundamentals. You can download the Selina Concise Biology ICSE Solutions for Class 10 with Free PDF download option. Selina Publishers Concise Biology for Class 10 ICSE Solutions all questions are solved and explained by expert teachers as per ICSE board guidelines.
Download Formulae Handbook For ICSE Class 9 and 10
ICSE SolutionsSelina ICSE Solutions
Selina ICSE Solutions for Class 10 Biology Chapter 3 Genetics – Some Basic Fundamentals
Exercise 1
Solution A.1.
d) Ascaris
Solution A.2.
a) 3 : 1
Solution B.1.
(a) – (iii) Study of laws of inheritance of characters
(b) – (v) Chromosomes other than the pair of sex chromosomes
(c) – (iv) A gene that can express when only in a similar pair
(d) – (ii) The alternative forms of a gene
(e) – (i) Chromosomes similar in size and shape
Solution B.2.
Lion, tiger, domestic cat (Any two)
Solution B.3.
Colour-blindness, Thalassaemia, Sickle cell anaemia and Haemophilia (Any two)
Solution B.4.
Homozygous dominant – RR
Homozygous recessive – rr
Solution C.1.
(a)
| Phenotype | Genotype |
| The observable Characteristic which is genetically controlled is called phenotype. | The set of genes present in the cells of an organism is called its genotype. |
(b)
| Character | Trait |
| Any heritable feature is called a character. | The alternative form of a character is called trait. |
(c)
| Monohybrid cross | Dihybrid cross |
| It is a cross between two pure breeding parent organisms with different varieties taking into consideration the alternative trait of only one character. | It is a cross between two pure breeding parent organisms with different varieties taking into consideration the alternative trait of two characters. |
Solution C.2.
The characteristics of a species such as physical appearance, body functions and behavior are not only the outcome of chromosome number, but these depend on the genotype of every organism. That means the set of genes present in the organisms may very and therefore lion, tiger and domestic cat have the same number of 38 chromosomes, their characteristics (like different appearances) are the result of the genes located on the chromosomes.
Solution C.3.
| Character | Dominant trait | Recessive trait |
| Flower Colour | Purple | White |
| Seed Colour | Yellow | Green |
| Seed Shape | Round | Wrinkled |
| Pod Shape | Inflated | Constricted |
| Flower Position | Axial | Terminal |
(Any 3)
Solution C.4.
Solution C.5.
Phenotypic Ratio – 3 (Black Fur) :1 (Brown Fur)
Genotypic Ratio – 1(Homozygous Black Fur):2 (Heterozygous Black Fur): 1 (Homozygous Brown Fur)
Solution D.1.
(a) Heterozygous: The condition in which a pair of homologous chromosomes carries dissimilar alleles for a particular character.
For example –
(b) Homozygous: The condition in which a pair of homologous chromosomes carries similar alleles for a particular character.
For example –
(c) Pedigree Chart: A pedigree chart is a diagram that shows the occurrence and appearance or phenotypes of a particular gene or organism and its ancestors from one generation to the next. In the pedigree chart, males are shown by squares and females by circles.
Solution D.2.
Mendel’s laws of inheritance are:
Solution D.3.

Solution E.1.

Solution E.2.
(a) Black
(b) No
Solution E.3.

Solution E.4.
(a) Father
(b) Two sons and three daughters
(c) The child 1 (daughter) is colour blind
(d) X chromosome
(e) Haemophilia
More Resources for Selina Concise Class 10 ICSE Solutions
ICSE Paper 2013
COMMERCIAL APPLICATIONS
(Two Hours)
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section A is compulsory. Attempt any four questions from Section B.
The intended marks for questions or parts of questions are given in brackets [ ].
Section-A (40 Marks)
(Attempt all questions from this Section)
Question 1:
Give one difference between each of the following:
(a) Industrial goods and Consumer goods. [2]
(b) Fixed assets and Current assets. [2]
(c) Sales promotion and Publicity. [2]
(d) Fixed deposit and Recurring deposit. [2]
(e) Direct labour costs add Indirect labour costs. [2]
Answer:
(a)
|
Industrial Goods |
Consumer Goods |
|
It is not directly consumed by the customer, but is used in the production of some other product. e.g. – Machine |
It is directly consumed by the ultimate consumers. e.g. — Soap. |
(b)
|
Fixed Asset |
Current Asset |
|
Fixed Assets are assets which are acquired to use in the organization for a long period of time to help in the servicing or manufacturing process. The duration of fixed assets'” is normally more than 5 years. |
Current assets are an asset which can be easily convertible into rupees. It is also used to meet the daily expenditure of the company. The duration of current assets is normally 1 to 2 years. |
(c)
|
Sales Promotion |
Publicity |
|
It refers to all those activities other than advertising that enhances consumer purchasing activities. These are non-recurring in nature. e.g. — Distribution of free samples. |
Publicity means non-personal means of communication which has no sponsor and is not paid by any organisation. e.g. — Audio-Video material. |
(d)
|
Fixed Deposit |
Recurring Deposit |
|
Fixed deposits are made (Single instalment) for a fix period and cannot be withdrawn before the expiry of the period for which they have been deposited. The rate of interest depends upon the period of time. |
In this the depositer is required to deposit a fixed amount every month for a fixed period of time. |
(e)
|
Direct Labour Costs |
Indirect Labour Costs |
| Labour which is directly consumed in the process of production. It is also known as productive or operating labour.
e.g.—Wages |
Labour which indirectly helps in the production process, which cannot be easily traced. e.g. —Salesman Salary. |
Question 2:
(a) What is Food Adulteration? Give an example. [2]
(b) Mention the elements of Public Relations. [2]
(e) Why should an Income and Expenditure Account be prepared? [2]
(d) Give two basic differences between Informaive advertising and Persuasive advertising. [2]
(e) What are ‘contingent liabilities’? [2]
Answer:
(a) Food Adulteration: It means deliberate mixing of foreign particles of low quality which are undesirable in the food items, to increase the quantity.
e.g. —Mixing of stone pieces identical to Wheat, Rice and Pulses.
(b) Public Relation Elements: (1) Human Relations, (2) Empathy, (3) Persuasion, (4) Dialogue.
(c) Income and Expenditure account is prepared to know the surplus or deficit arising from the activities of a non-trading concern during a year.
(d)
|
Informative Advertising |
Persuasive Advertising |
|
1. Informative advertising is commonly used to drive “primary demand” for new product and service categories. |
1. The goal of persuasive advertising is to drive selective demand for specific products or services. |
| 2. It is used to emphasize the product name, product benefits and its possible uses to the prospective market. |
2. It is used after product introduction to customers. Its main goal is to enhance selective demand for the product. |
(e) Contingent Liabilities: These are those liabilities which become payable on the’happening of an event.
e.g. — bills discounted but not matured, guarantee for a loan. These are also known as anticipated liabilities.
Question 3:
(a) On the basis of ownership, distinguish between a Product and a Service. [2]
(b) Explain in brief the term ‘Parity Princing’. [2]
(c) How does the Central Bank control credit through Statutory Liquidity Ratio. [2]
(d) Explain the terms Surplus and Deficit in an Income and Expenditure Account. [2]
(e) With reference to the Bhopal Gas Tragedy:
(i) Name the company responsible for the tragedy.
(ii) Identify the poisonous gas that caused this ghastly man made disaster. [2]
Answer:
(a)
|
Product |
Service |
|
Ownership of a product is transferable. e.g. — When a T.V. is bought, the ownership is transferred from the seller to a buyer. |
Ownership of service is not transferable. e.g. — Taking house on a rent, won’t transfer the ownership from the seller to you. |
(b) Parity Pricing: Parity Pricing concept that the selling price of a product or produce should.go up in the same account as the peices of the inputs used in product. It means adjustment of price according to the competitors. It is best suitable for a highly competitive market.
(c) Statutory Liquidity Ratio (SLR): Statutory liquidity ratio refer amount that the commercial banks require to maintain in the form of gold or government approved securities before providing credit to the customers. SLR is determined and maintained by the Reserve Bank of India in order to control the expansion of bank credit.
(d) Surplus: If the income of a non-trading concern exceeds over its expenditure, it is known as surplus.
Deficit: If the expenditure exceeds the income, it is known as deficit.
(e) (i) The company responsible for the Bhopal Gas Tragedy – Union Carbide India Limited.
(ii) Methyl Isocyanate (MIC).
Question 4:
Justify either for or against by giving two reasons for each of the following:
(a) Aggressive selling is the only way a company can survive in a highly competitive market. [2]
(b) Real costs must be recorded in the books of accounts. [2]
(c) Training reduces employee turnover. [2]
(d) Tea and coffee can be sold on sale-on approval or return basis. [2]
(e) Increased advertising in recent times has resulted in lower prices of newspapers. [2]
Answer:
(a) For: Yes, it is true because through agressive selling only you can get more customers to exchange their money for your products and services by proving yourself superior among the competitors. To achieve this, new marketing and selling techniques are used to explore the needs of customers, to get their maximum satisfaction.
(b) Against: Real cost cannot be recorded in the books of accounts because value of this cannot be measured in terms of money. It means sacrifice, discomfort and pain involved in doing the business.
(c) For: Yes, training reduces employee turnover as it increases the efficiency of worker which boosts the morale of the employees. This ensures employee loyalty, long term growth and a lifetime association with the organisation.
(d) Against: Tea and coffee cannot be sold or sale on approval or return basis because under this arrangement of sale an individual or a company interested in purchasing a specific item is allowed to use the item for a given length of time. At the end of that time if the individual is satisfied with the item they agree to purchase otherwise it has to be returned, but in the case of tea and coffee nothing could be returned, after the consumption (if not satisfied) and if, consumed partially it will be difficult to do the valuation of the consumed portion.
(e) For: Yes, increased advertising in recent times has resulted in lower prices of newspapers, as now a days advertising has become a great source of income for newspapers which has resulted in increase in its circulation.
Section – B (60 Marks)
(Attempt any four questions from this section)
Question 5:
(a) What are Overheads or Indirect expenses? Mention three types of Overheads with an example of each. [5]
(b) ‘Intense competition in the corporate world has led to the emergence of advertising as a vital tool for corporate survival’. Do you agree with this statement? Support your answer by citing reasons. [5]
(c) Explain the following:
(i) Principle of Prudence.
(ii) Money Measurement Concept. [5]
Answer:
(a) Overhead or indirect expenses are those expenses which cannot be charged to production directly or cannot be identified with a specific product or job. These are incurred in planning and controlling the business operations.
Three types of overhead expenses are:
(b) Intense competition in the corporate world has led to the emergence of advertising as a vital tool for corporate survival because these days, there are several producers producing similar products, therefore the primary objective of advertising is to provide relevant information to the consumers about products and services. It also provides link between producers and consumers. With the help of convincing advertisement, a business firm can create demand for its products which facilitates mass distribution of goods. By creating brand loyalty, advertising helps to maintain sales and market-share.
(c) (i) Principle of Prudence: According to this convention all anticipated losses should be recorded in the books of accounts, but all anticipated or unrealized gains should be ignored, e.g. – Joint Life Insurance Policy is shown only at surrender value as against the amount paid.
(ii) Refer Ans. 8. (a) (ii), 2016.
Question 6:
(a) What is the Penetrating Princing Policy? Discuss its pros and cons. [5]
(b) Elucidate the selection procedure for a vacancy in an organizaiton. [5]
(c) Briefly explain any five Consumer Rights. [5]
Answer:
(a) Penetrating Pricing Policy: According to this pricing policy, in the initial stage low prices are set-up to make the particular brand popular. This strategy is mostly used when there is strong potential competition in market and while launching fast moving consumer goods where demand is highly elastic and to restrict the entry of new competitors in the market. But at the same time the demand goes so high that it becomes difficult for the producer to meet it or the consumers think that the low price product are low in quality.
Pros:
Cons:
(b) Selection Procedure:
(c) Consumer Rights:
Question 7:
(a) Describe the procedure adopted for opening a Saving Bank Account with a Commercial Bank. [5]
(b) Briefly explain any five factors responsible for the destruction of the eco system. [5]
(c) List the advantages and disadvantages of using radio services as a form of advertising media. [5]
Answer:
(a) Procedure for Opening a Saving Bank Account:
(b) Five Factors Responsible for the Destruction of the Eco System:
(c) Advantages of Radio Services in the form of Advertising Media:
Disadvantages:
Question 8:
(a) What is the professional and social significance of Human Resource Management? [5]
(b) Write short notes on:
(i) Importance of Packaging.
(ii) The Chernobyl disaster. [5]
(c) Last year, Sakona Co. Ltd. came up with a unique and revolutionary Home Theatre System with amazing new features, excellent sound quality and elegant design. It was priced very high, yet it was a great success. This year the company is facing stiff competition, but is determined to acquire a huge chunk of the market share.
(i) In what stage of PLC (Product Life Cycle) is the Home Theatre System presently?
(ii) Suggest strategies that Sakona Co. Ltd. should adopt to achieve its corporate goals. [5]
Answer:
(a) Professional and Social Importance of Human Resources Management:
Professional Significance: Human Resource Management helps in maximising the working potential of the employees by providing a healthy working environment and opportunities for growth. It enhances better understanding among the people working together which leads to effective team work. It boosts the confidence of the people by placing them at the right place at the right time according to their qualification and work experience. HRM also inculcates a feeling of belonging for the organisation.
Social Significance: Human Resource Management helps the society by utilising and channelising the manpower in the productive channels. It provides maximum opportunities to enhance the dignity of labour by placing the right person at the right job. HRM also raises the standard of living of the people by increasing the employment opportunities.
(b) (i) Packaging: It means placing products in suitable packages for safe and easy handling. It is not only a protective cover but it also acts as a silent salesman by providing various information about the product. Packaging also provides reuse, repack and resale value of the packaging material which adds utility to the product. It also has a competitive value as it differentiates a product from its substitutes and adds beauty to the product to grab immediate attention.
(ii) The Chernobyl Disaster: The Chernobyl disaster occurred on April 26, 1986 at Chernobyl nuclear plant located in Chernobyl city of Ukraine. It is a nuclear power generation station from where radioactive material drifted over the surrounding area causing more than 10,000 people died due to side effect of radiation. It lead to a series of genetic disorders or changes in the areas of Ukraine, Belrus and Russia.
(c) (i) At present the PLC of the Home Theatre System is in its growth stage where it is facing a tough competition. Still the sale at distribution is widening.
(ii) At this stage the Sakona Co. Ltd should adopt the following strategies:
Question 9:
Case Study
It is the conviction of the management of Carmel Closure Co. Ltd. that its shareholders constitute an important part of the public. The management feels that the company should devote time and effort in studying the make-up, location, economic status and demography of this group to enable better communication and a stronger public relation policy.
Mr. Ramesh Sharma is the Public Relation Officer of the company. He agrees with the Management to a certain extent, however he would like to introduce other forms of public relations programmes.
(a) Why is it imperative for a company to maintain a good relationship with its shareholders?
How should Mr. Sharma go about planning a successful programme? [5]
(b) Mention two other public relations programmes that would be of paramount importance to the company. Give reasons to support your answer. [5]
(c) How would you explain to Mr. Sharma about the element ‘Empathy’ as being one of the key elements to establish good public relations? [5]
Answer:
(a) It is important for a company to maintain a good relationship with its shareholders because they are the one who contributes to the company’s share capital and assure the risk of the loss. The company should consider the shareholder as its actual owner and should always strive for a better relationship with its shareholders to hold their interest in the company and j promote holding of stock as a long term investment.
Mr. Sharma should provide the current information about the financial position and future prospectsnf the company in a simple and under-standable language. The company should organise share holder relation programmes to promote the long-term investment relationship with the company. This will reduce the dissatisfaction and organised opposition to management policies.
(b) The two public relation programmes that would paramount importance to the company are:
(c) Empathy means looking at things from others point of view which leads towards better understanding of another human being. It is basically concerned with the establishment of trust and rapport, which is the base for public relation. It involves seeing and feeling matters as others see and feel which builds more confidence of the public and promotes goodwill of the company. It also appreciate company’s perspective in a positive way by creating an atmosphere full of faith, trust and confidence.
Question 10:
Case Study
Ardent Aptec Ltd. is a telecommunication company. It gives top priority to employee training. The company believes that training contributes to a permanent relationship between superiors and subordinates. The company environment provides conditions favourable to learning and career growth. It also considers it as a continuous process.
The recruitment policy of the company is focused on campus recruitment and hence special steps are taken to ensure that the employees acquire the necessary skills. The accelerated rate of technological changes in the telecommunication industry has led to greater focus on retraining employees.
In addition to in-house training facilities, the company makes use of training programmes run by various technical and management institutes.
(a) Discuss any three types of training that can be undertaken by Ardent Aptec Ltd. [5]
(b) Why does Ardent Aptec Ltd. think it is important to train employees? [5]
(c) Discuss any three training methods that may be adopted by Ardent Aptec Ltd. [5]
Answer:
(a) The three types of training that can be undertaken by Ardent Aptec Ltd are as follows:
(b) Ardent Aptec Ltd, thinks training is very important because training is a learning experience which develops manpower to improve their job performance. It also helps workers to cope with the latest technological advancement taking place rapidly which boosts the morale of the workers to face the complex situations at the work place. Well trained employees require less supervision which reduces the cost of supervision and increases the probability of expansion of work.
(c) Three training methods that may be adopted by Ardent Aptec Ltd. are:
APlusTopper.com provides ICSE Class 10 Economic Applications Previous Year Board Question Papers Solved Pdf Free Download with Solutions, Answers and Marking Scheme. Here we have given ICSE Class 10 Economic Applications Solved Question Papers Last Ten Years. Students can view or download the ICSE Board 10th Economic Applications Previous Year Question Papers with Solutions for their upcoming examination.
These ICSE Class 10 Economic Applications Previous 10 Years Board Question Papers with Answers are useful to understand the pattern of questions asked in the board exam. Know about the important concepts to be prepared for ICSE Class 10 Board Exam and Score More marks.
Board – Indian Certificate of Secondary Education (ICSE), www.cisce.org
Class – Class 10
Subject – Economic Applications
Year of Examination – 2020, 2019, 2018, 2017.
We hope the ICSE Class 10 Economic Applications Previous Years Question Papers Solved Last 10 Years Pdf Free Download with Solutions will help you. If you have any query regarding ICSE Class 10 Economic Applications Solved Question Papers Last Ten Years with Answers, drop a comment below and we will get back to you at the earliest.
APlusTopper.com provides ICSE Class 10 English Literature Previous Year Board Question Papers Solved Pdf Free Download with Solutions, Answers and Marking Scheme. Here we have given ICSE Class 10 English Literature Solved Question Papers Last Ten Years. Students can view or download the ICSE Board 10th English Literature Previous Year Question Papers with Solutions for their upcoming examination.
These ICSE Class 10 English Literature Previous 10 Years Board Question Papers with Answers are useful to understand the pattern of questions asked in the board exam. Know about the important concepts to be prepared for ICSE Class 10 Board Exam and Score More marks.
Board – Indian Certificate of Secondary Education (ICSE), www.cisce.org
Class – Class 10
Subject – English Literature
Year of Examination – 2020, 2019, 2018, 2017.
We hope the ICSE Class 10 English Literature Previous Years Question Papers Solved Last 10 Years Pdf Free Download with Solutions will help you. If you have any query regarding ICSE Class 10 English Literature Solved Question Papers Last Ten Years with Answers, drop a comment below and we will get back to you at the earliest.
APlusTopper.com provides ICSE Class 10 Hindi Previous Year Board Question Papers Solved Pdf Free Download with Solutions, Answers and Marking Scheme. Here we have given ICSE Class 10 Hindi Solved Question Papers Last Ten Years. Students can view or download the ICSE Board 10th Hindi Previous Year Question Papers with Solutions for their upcoming examination.
These ICSE Class 10 Hindi Previous 10 Years Board Question Papers with Answers are useful to understand the pattern of questions asked in the board exam. Know about the important concepts to be prepared for ICSE Class 10 Board Exam and Score More marks.
Board – Indian Certificate of Secondary Education (ICSE), www.cisce.org
Class – Class 10
Subject – Hindi
Year of Examination – 2020, 2019, 2018, 2017.
We hope the ICSE Class 10 Hindi Previous Years Question Papers Solved Last 10 Years Pdf Free Download with Solutions will help you. If you have any query regarding ICSE Class 10 Hindi Solved Question Papers Last Ten Years with Answers, drop a comment below and we will get back to you at the earliest.